The Nuclear Medicine Section is taking part in a new clinical trial to test the effectiveness of a novel molecular imaging agent to improve the diagnosis of brain metastasis recurrence after radiation therapy in cancer patients. The phase II PURSUE multi-center clinical trial evaluates the role of a novel amino acid tracer in non-invasive detection of tumor recurrence in patients with brain metastases. The Yale team is the first in the United States to recruit patients to this trial, which will establish the criteria for interpretation of positron emission tomography (PET) imaging using the novel amino acid tracer in brain metastasis evaluation.
The researchers will test the effectiveness of the diagnostic imaging agent, 18F-fluciclovine (Axumin® [fluciclovine F 18]), for diagnosing tumor recurrence in patients with brain metastases who have undergone radiation therapy. The researchers are from the Nuclear Medicine and Neuroradiology sections of the Department of Radiology & Biomedical Imaging, and the Department of Neurosurgery at Yale School of Medicine (YSM).
“Axumin is FDA-approved for evaluation of suspected recurrence of metastatic prostate cancer,” said Mariam Aboian, MD, PhD, who is leading the study for the Department of Radiology & Biomedical Imaging. “18F-fluciclovine is a unique tracer because it has very low uptake in normal brain tissue, which makes it an excellent imaging method to study central nervous system malignancies,” Dr. Aboian said.
The researchers, with the help of YCCI, are working with the molecular imaging diagnostics company, Blue Earth Diagnostics to launch this multi-center clinical trial at YSM. The controlled study will test the safety and efficacy of 18F-fluciclovine for use in brain metastasis. The results of the study are planned to be submitted, alongside other data, to the FDA for potential approval after studies are completed.
Positron emission tomography (PET) is a method to image molecular processes in the tissue, using a tracer to label the tissue. “18F-fluciclovine is an amino acid that is labeled with the radionuclide fluorine-18 that allows us to visualize it using PET. As cancer cells consume increased levels of nutrients such as amino acids, very small amounts of 18F-fluciclovine may be used to label cancer cells, and this allows us to visualize those cancer cells on medical images,” Dr. Aboian explained.
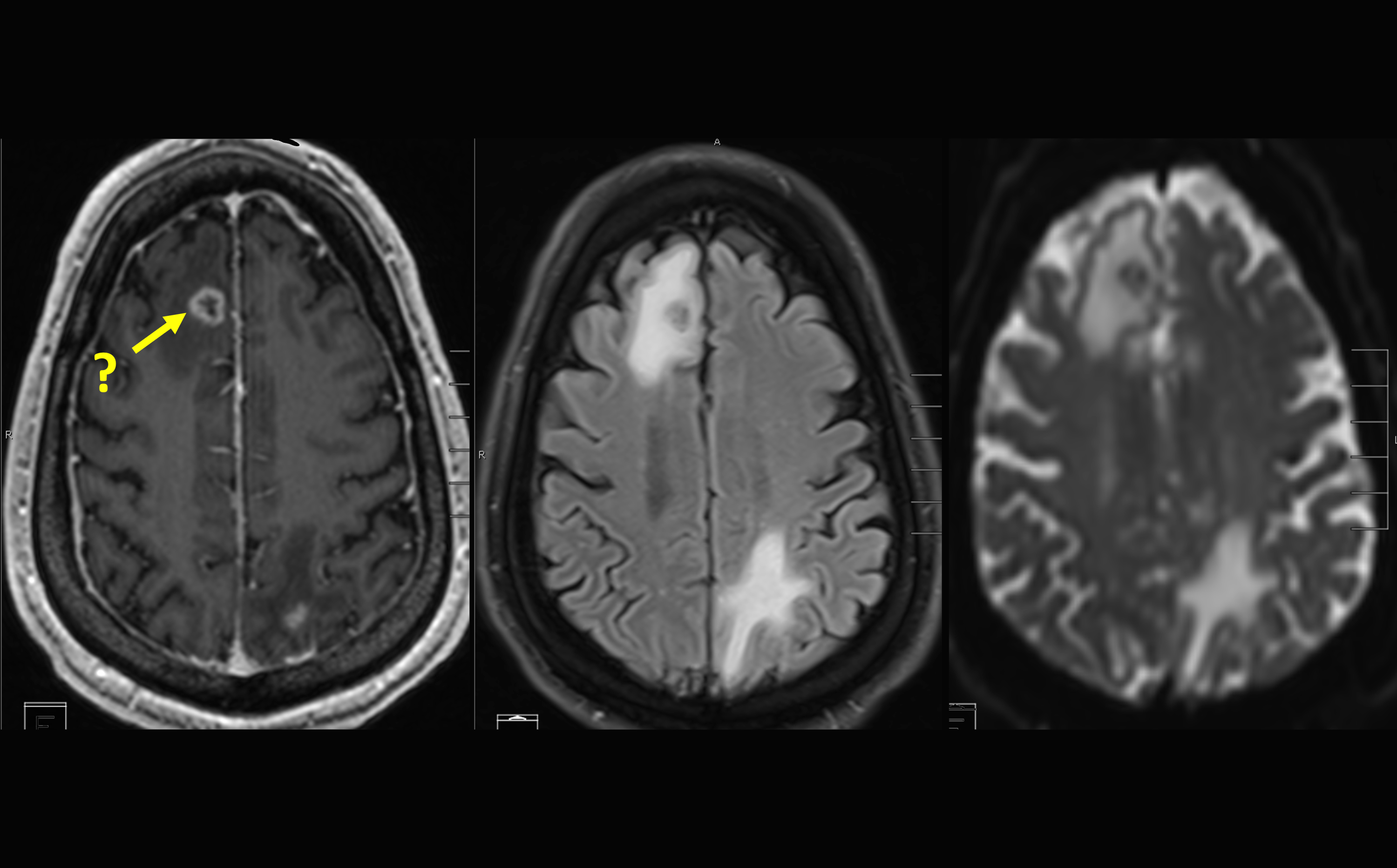
Pending results from these studies and potential FDA approval, 18F-fluciclovine could be the first commercially available tracer for the evaluation of brain metastasis after radiation therapy to help distinguish tumor recurrence from radiation necrosis – posttreatment changes in normal brain tissue.
‘Initial diagnosis of brain metastatic disease often depends on magnetic resonance imaging (MRI). However, radiation necrosis and a recurrent tumor can appear similar on an MRI, which is why 18F-fluciclovine would be a valuable diagnosis tool,” said Lawrence Saperstein, MD, Chief of the Nuclear Medicine Section.
“The potential benefit to patients would be invaluable,” Dr. Aboian said. “If we diagnose tumor recurrence, the patient can undergo surgery more quickly, without waiting to confirm with follow up imaging. And if it’s not recurrence, they get to avoid unnecessary surgery and go on with their lives.”
Also leading the trial at YSM is Veronica Chiang, MD, professor of neurosurgery, who directs the neurosurgical arm of the Brain Metastasis Program at Yale. “I think that amino acid PET might have the potential to enable us to differentiate tumor from other non-malignant pathologies either at initial diagnosis or after first-line treatment with radiation or surgery,” said Dr. Chiang. “The biggest clinical need for this differentiation today is separating re-growing tumor from radiation necrosis - which is the goal of the PURSUE trial.”
“Molecular imaging can be very sensitive for certain cancers, such as prostate or neuroendocrine tumors, and may detect disease sites that are hidden on conventional CT or MR imaging,” said Darko Pucar, MD, PhD, co-director of the newly formed Molecular Imaging Clinical and Translational Research Laboratory (MICTRL). Axumin is currently FDA-approved for recurrent prostate cancer. Molecular Imaging agents can detect cell receptors, such as Prostate Specific Membrane Antigen (PSMA), or abnormal cellular metabolism, such as 18F-fluciclovine,”.
“Radiation therapy is a very effective treatment for patients with lung, breast, or melanoma metastasis to the brain,” Dr. Aboian said. “For those patients, it’s really helpful to know if the metastasis is recurrent in the setting of lesion that is growing in size. We are excited to be involved in the early stages of these trials to set up efficient workflows and expertise in using these novel imaging approaches.”
“We want patients to know that we have leading research on imaging tracers. Right now, it’s on a trial basis, but after we conduct this trial, and 18F-fluciclovine hopefully receives FDA approval for this new indication, we will be the experts in terms of imaging patients with this technique,” she added.
“When we have a diagnostic test that will give us a definitive and accurate prediction of whether its radiation necrosis or tumor recurrence, that’s going to help a lot of patients.”
Featured in this article
- Darko Pucar, MD, PhDAssociate Professor of Radiology and Biomedical Imaging; Co-Director of Molecular Imaging Clinical and Translational Research Laboratory, Nuclear Medicine; Yale Radiology Thesis Committee Chair, Clinical Radiology; Yale Nuclear Radiology Residency Pathway Director, Nuclear Medicine